CPT Infrastructure:
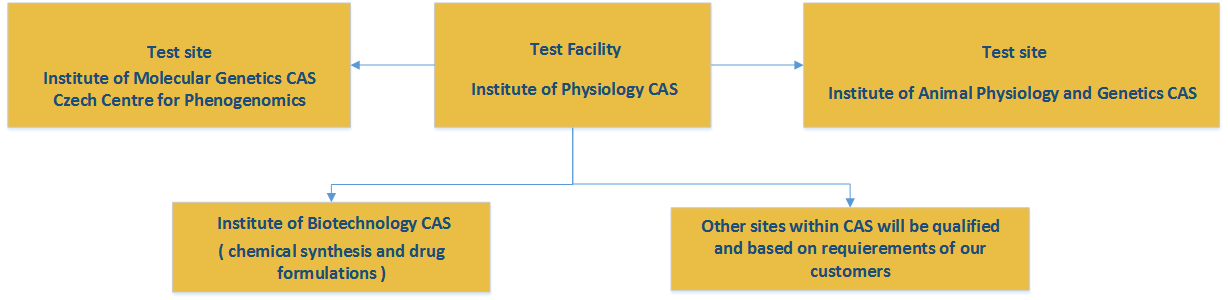
Our preclinical platform comprise the following institutes:
Institute of Physiology CAS: test facility, GLP certified, responsible for toxicity testing on rodents (mouse, rat, guinea pig) and non-rodents (rabbit), including hematology, http://www.fgu.cas.cz/
[ezcol_1quarter] [/ezcol_1quarter]
[ezcol_1quarter]
[/ezcol_1quarter]
[ezcol_1quarter] [/ezcol_1quarter]
[ezcol_1quarter]
[/ezcol_1quarter]
[ezcol_1quarter] [/ezcol_1quarter]
[ezcol_1quarter_end]
[/ezcol_1quarter]
[ezcol_1quarter_end] [/ezcol_1quarter_end]
[/ezcol_1quarter_end]
Institute of Molecular Genetics CAS, Czech Centre for Phenogenomics: test site, established quality system, responsible for histopathology, clinical chemistry, hematology, bioanalytical testing*, determination of metabolites, specific toxicity studies, imaging, immunology, neurophysiology, pharmacology on xenografts;
*bioanalytical testing can be performed either exclusively by IMG or in collaboration with our partner, company Pharmakl spol. s r.o.
http://www.phenogenomics.cz/phenotyping/
[ezcol_1fifth] [/ezcol_1fifth]
[ezcol_1fifth]
[/ezcol_1fifth]
[ezcol_1fifth] [/ezcol_1fifth]
[ezcol_1fifth]
[/ezcol_1fifth]
[ezcol_1fifth] [/ezcol_1fifth]
[ezcol_1fifth]
[/ezcol_1fifth]
[ezcol_1fifth] [/ezcol_1fifth]
[ezcol_1fifth_end]
[/ezcol_1fifth]
[ezcol_1fifth_end] [/ezcol_1fifth_end]
[/ezcol_1fifth_end]
Institute of Animal Physiology and Genetics CAS: test site, established quality system, responsible for toxicity studies on non-rodents – minipigs, http://www.iapg.cas.cz/sekce&id=7
[ezcol_1fifth]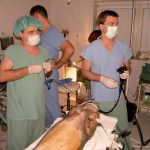 [/ezcol_1fifth]
[ezcol_1fifth]
[/ezcol_1fifth]
[ezcol_1fifth] [/ezcol_1fifth]
[ezcol_1fifth]
[/ezcol_1fifth]
[ezcol_1fifth] [/ezcol_1fifth]
[ezcol_1fifth]
[/ezcol_1fifth]
[ezcol_1fifth] [/ezcol_1fifth]
[ezcol_1fifth_end]
[/ezcol_1fifth]
[ezcol_1fifth_end] [/ezcol_1fifth_end]
[/ezcol_1fifth_end]
Institute of Biotechnology CAS: responsible for custom chemical synthesis of promising therapeutic substances, development of application formulations), http://www.ibt.cas.cz/core-facility/Service_Technology_lab.html

